Going to the market at the beginning of the day produces many options, most of which are of high quality and plentiful. Unfortunately, by the end of the day, the options are no longer bountiful or beautiful. The same principle applies to sub-human species including our close cousins, the primates. Many foragers live by learning how best to exploit unpredictable seasons of preferred food items. For example, consider the quest for figs by orangutans. These foragers must be able to maximize their various foraging opportunities across the year when preferred resources may be sparse. Feast or famine – in the case of orangutans, they have been known to gorge on all the figs they can find while at other times they choose the leaner diet of bark and leaves.

What to do?
Understanding the behavioral ecology, or the behavioral responses of animals to their natural habitat as they eat, mate, protect, and care for young, is critical in designing accurate experimental paradigms or studies that can test specific hypotheses. For example, we have learned that orangutans will forage for different types of foods depending on the time of year, season, previous rains, and available tools. Most of this knowledge has come from researchers following orangutans in their natural habitat.
This fieldwork has raised the question, how do orangutans “know” this information? In particular, how do orangutans formulate their decisions considering the number of factors they must process simultaneously within a short period of time (e.g., which tree is fruiting next? How many other orangutans are in the area that must be competed with? Do I have enough energy to move to the next patch that might be a distance away? What is that distance?).
These types of questions are best answered in a laboratory setting, especially if experimenters can design a task that is relatively natural or simulates a natural behavior. For example, in a previous post, I discussed the paradigm of marble burying by mice as an animal model of compulsive or anxiety-based behavior. Although the marble-burying paradigm seemed to elicit more inherent behaviors of curiosity in mice rather than being a good model for eliciting compulsive/anxiety-like behaviors, this artificial paradigm might be a valid proxy for individuals with certain extreme characteristics. More research is still needed to unravel the question of this specific paradigm and its implications.
Foraging at its best. What’s behind it?
This post focuses on a recent article published in the Psychonomic Society’s journal Cognitive, Affective, and Behavioral Neuroscience.  Researchers Sam Hall-McMaster and Fabrice Luyckx of the University of Oxford conducted a theoretical review in which they examined current laboratory paradigms that simulate foraging decisions from a neuroscience perspective.
Researchers Sam Hall-McMaster and Fabrice Luyckx of the University of Oxford conducted a theoretical review in which they examined current laboratory paradigms that simulate foraging decisions from a neuroscience perspective.
Marrying the ability to predict foraging behavior (i.e., another line of evidence to investigate reinforcement learning or economic task choices) with an examination of the neural connections as these decisions are made requires highly controlled environments with the ability to evaluate the neural response as it is happening (e.g., fMRIs in the case of humans or implanted neuron recorders in the case of rats or primates).
Foraging behavior requires a number of decisions in which risks and benefits are weighed by the foraging individual. When an individual is able to maximize reward while minimizing effort, and while considering the current food source option with recent past options, Optimal Foraging Theory is in operation. Optimal Foraging Theory assumes that the most economically advantageous foraging pattern will eventually emerge in a species through evolutionary pressures.
Take the image below. . .

Although not representative of most of the laboratory studies testing the Optimal Foraging Theory hypotheses, this DIY foraging task made up of toilet paper rolls and unequal spacing, requires the rat to make choices. Imagine the rat moving around the foraging area (i.e., the differently spaced toilet paper rolls). Depending on its previous experiences with this foraging patch, the rat has to make some choices: (1) “should I stay or leave because the patch is getting sparse” (i.e., stay-switch or patch-leaving), or (2) “do I go ahead and eat now or should I wait because something better might come along” (i.e., accept-reject or diet-selection)?
All for one, or one for all?
These choices are initially driven through trial and error as is predicted by reinforcement learning, in which individuals have to learn to maximize future rewards over time through various experiences. If other factors are involved, such as the presence of another individual while foraging, then an individual rat must now decide if it should maximize its individual success (eat until full, and maybe exploit the resource) or work with the other rat to maximize overall success (eat enough to move to another patch or eat until nothing is left but both rats are full).
This choice simulates an economic choice task often confronted by humans, such as the Prisoner’s dilemma, in which partners must decide to take a smaller reward if they choose not to cooperate vs. cooperating with each other and gaining a bigger reward.
Shall we eat in or dine out tonight?
The foraging approach is characterized by two features: (1) a reward quantity must be maximized in concert with another variable such as time or effort expended (i.e., how can the orangutan get the most food based on distance traveled), and (2) one option is currently available (i.e., foreground option, the fig tree is ripe) while another option is available but not pursued simultaneously (i.e., background option, another tree has figs that aren’t fully ripe yet). Unfortunately, much of the earlier research on foraging did not allow subjects to direct their encounters. Although controlling encounters at food sources may be appealing to experimenters (i.e., animals can’t go back to a source once they have visited it or once one toilet paper roll has been investigated, it is no longer in play), this experimental paradigm likely does not allow the animals to demonstrate their foraging strategies properly.
Au Naturel?
Hall-McMaster and Luyckx proposed that to simulate a more naturalistic foraging experience future studies should incorporate several important changes: (1) animals should be able to direct their encounters with choice items, (2) animals should be able to revisit previously foraged locations, (3) patches should replenish at different rates, and (4) patch distributions should be manipulated. These changes would enable the neuroscience side to discriminate between which of the two strategies (i.e., stay-switch or accept-reject) is being implemented during foraging decisions.
World domination at its finest
Currently, two neural paths seem to be involved in decision-making, especially in situations in which rewards such as food are involved. The dorsal anterior cingulate cortex (dACC, the pink area in the human brain image below) and the ventromedial prefrontal cortex (vmPFC, green area in the human brain image) have both been implicated in foraging tasks. As represented in the schematic below, both areas influence each other as summarized by Hall-McMaster and Luyckx.
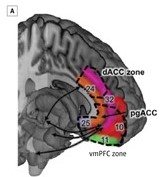
Based on a series of primate studies, the dACC seems to be involved with decisions to leave a patch, which seems to be related to the time it would take to get to the next patch and whether or not the next patch is as good as the current patch or past patches.
The vmPFC seems to process the reward magnitude of the chosen foraging option. When activity in both regions is considered, the results suggest that these two areas may interact to compare the current foraging option with the relative value of searching for alternatives.
More recent research involving the manipulation of specific neurons in areas around the dACC has shown that rats can be manipulated to make switch decisions earlier and more frequently.
Currently, two competing functional explanations exist regarding the dACC’s function: (1) it plays an important role in behavioral persistence and flexibility, and (2) it functions to direct one’s cognitive control and decide how much control is needed. By making foraging paradigms more naturalistic, Hall-McMaster and Luyckx hope that the neural underpinnings of decision making can be uncovered more clearly as more testable predictions can be established. Whether it is making a decision about where the best foraging opportunities would be or how to dominate the world if one was the Brain from Pinky and the Brain, it is clear that much more work is needed and more naturalistic experimental paradigms are the key.
Psychonomics article focused on in this post:
Hall-McMaster, S., & Luyckx, F. (2019). Revisiting foraging approaches in neuroscience. Cognitive, Affective, & Behavioral Neuroscience, 19, 225-230. DOI: 10.3758/s13415-018-00682-z.
